Identification of Rubber polymer
Needed Glassware
Hard glass Tube,Bent glass tube with cork stopper, Test tube
Hard glass Tube,Bent glass tube with cork stopper, Test tube
Required chemical Reagents:-
Solution I –
Dissolve 1.0 g of p-dimethylamine benzaldehyde and 10 mg of hydroquinone in 100 ml of methanol. Add 10 ml of ethylene glycol and 5 ml of concentrated hydrochloric acid. Dilute the reagent with methanol to obtain relative density of 0.85 at 25°C.
Solution II –
Dissolve 2.0 g of sodium citrate crystals 200 mg of citric acid and 30 mg each of indicators bromocresol green and metanil yellow in 500ml of water.
Procedure –
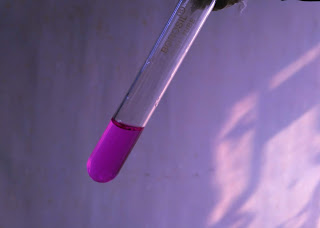
Place 0.5 to 2.0 g of sample in a hard glass tube equipped with a bent glass condensing tube of about 4 mm bore by means of cork stopper. Heat the test tube on a Bunsen burner. When the sample starts decomposing immerse the other end of condensing tube into a test tube containing Solution II. If a red colour develops then it confirms
polychloroprene rubber. If no colour change is observed then pass the vapours into Solution I and note the colour change. Then add 5 cm3 of methanol and heat on a water bath and note if there is any colour change. shown in pic.
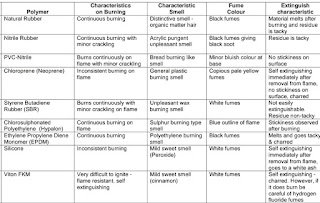
Burning Testing
Reference:- IS 3400 part 22


Absolutely with you it agree. It is excellent idea. It is ready to support you.
I think this is one of the most vital info for me. And i’m glad reading your article. But should remark on few general things, The web site style is wonderful, the articles is really nice : D. Good job, cheers