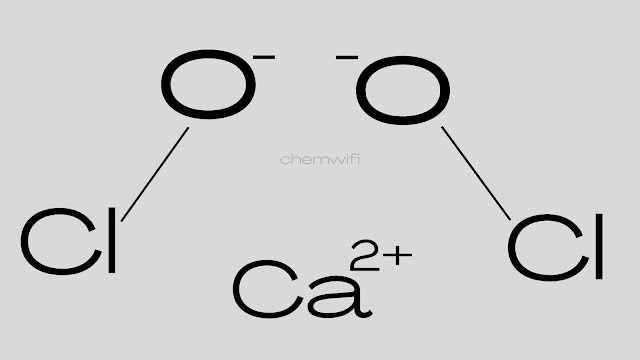
Bleaching Powder
Bleaching powder is calcium hypochlorite. it’s also called Hypochlorus acid calcium salt bleaching powder, calcium oxychloride or chloride of lime.
Properties of Bleaching powder
- Chemical formula of Bleaching powder – Ca(ClO)2
- Molecular weight of Bleaching powder – 142.98 g/mol
- Density of Bleaching powder – 2.35 g/cm³ at 20 ⁰C
- Melting point of Bleaching powder – 100 ⁰C
- Boiling point of Bleaching powder – 175 ⁰C
- Solubility in water – 21 g/100ml at 25 ⁰C
- Bleaching powder is a white powder which gives a strong smell of chlorine.
- Bleaching powder is soluble in cold water.
- Bleaching powder reacts with dilute acid (H2So4) to produce chlorine.
CaOCl2 ➕ H2So4 ➝ CaSO4 ➕ Cl2 ➕ H2O
Bleaching powder sulfuric Acid Calcium Sulfate Chlorine water
(dilute)
Preparation of bleaching powder
Bleaching powder is prepared by passing chlorine gas over dry slaked lime.
Ca(OH)2 ➕ Cl2 ➝ CaOCl2 ➕ H2O
Calcium Hydroxide Chlorine Bleaching powder water
(Slaked lime)
Uses of bleaching powder
Bleaching powder is used for the manufacture of Chloroform (CHCl3), making wool unsinkable, Oxidizing agent in many chemical industries, disinfecting drinking water supply. that’s for making drinking water free from germs.
-
it’s used for bleaching cotton, linen & other fiber in the textile industry and for bleaching wood pulp in the paper industry. it is also used for bleaching washed clothes in the laundry.