Control of Absorbance
Potassium Dichromate Solution:-
Weigh & transfer a quantity about 30 mg of previously dried Potassium Dichromate ( NIST ) ( at least 4 hour at 130 o C ) to 500 ml volumetric flask. Dissolve & dilute in sufficient 0.005M H2SO4 (136 μL) to produce 500 ml.
Blank 0.005M H2SO4 solution :-
Add 136 μL of concentrated sulfuric acid ( 98% H2SO4 specific gravity g 1.84) into a 500 ml volumetric flask containing 250 ml of purified water. Mix, cool and dilute to volume.
Go to Photometric mode
- Press Ctr + M
- Add wavelength 235,257,313 & 350 nm {Select RAW DATA}
- Make sure that Reference and sample cell holder is empty and press button for baseline correction(230 to 350 nm).
- Then keep the Potassium dichromate Solution in the sample cell holder & using 0.005 M H2SO4 solution as a blank
- Close the cell compartment
- Click Start
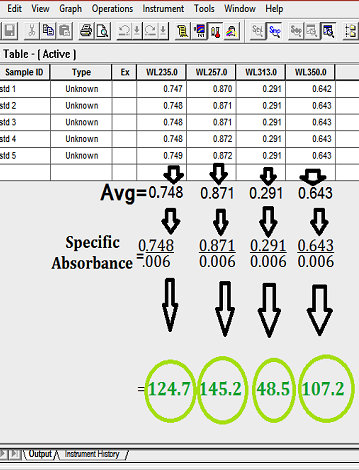
- Calculate value of A (1% 1 cm) for each wavelength and record it.
- Specific Absorbance = Observed Absorbance/ 0.006
Acceptance criteria:-
| Wavelengths (nm) | Limit |
|---|---|
| 235 | 122.9 – 126.2 |
| 257 | 142.8 – 145.7 |
| 313 | 47.0 – 50.3 |
| 350 | 105.6 – 108.2 |
 |
| CONTROL OF ABSORBANCE (UV calibration) K2Cr2O7 |
If calibration is satisfactory then affix the status label on it with the next due calibration date.
Frequency
Once in a 3 month